LASAG INHALE©
A Safe and Comfortable Pain and Migraine Therapy
Our Mission:
Changing Treatment Paradigms for
Severe Head Pain and Migraine
Who we are
AspiAir in Germany is a clinical-stage biotech company focusing on the development of safe and comfortable non-opioid treatments for pain and migraine attacks. Members of the former clinical and project management team of Vectura founded the privately financed startup in 2019. AspiAir has its main operations near Marburg and is backed by the family office ATON GmbH in Munich. AspiAir holds several formulation and device patents to protect the therapeutic approach.
The most advanced program is an inhaled version of LASAG (Acetylsalicylic Acid – ASA). It is the first non-invasive, mobile LASAG treatment – at home or on the road.

Management

Dr. Stefan Degenhardt
Managing Director & CFO
Stefan has over 20 years of experience in senior finance and management in the life science industry. He has played a key role during the sale of Activaero GmbH to Vectura plc in 2024. Stefan is Managing Director of AspiAir since 2020. He is also a Co-Founder of Activoris Medizintechnik GmbH.

Dr. Sebastian Canisius
Chief Medical Officer
Sebastian is an experienced physician focusing on pulmonary and sleep medicine clinical development including drug safety and pharmacovigilance. Sebastian runs his own consulting business ACliRA. At AspiAir, he overseas clinical trial design and study conduct.

Dr. Karlheinz Nocker
Project Manager
Karlheinz holds a PhD in chemistry and has over 20 years of experience in the pharmaceutical industry. He joined AspiAir in 2020 being responsible for the development of inhaled LASAG. Karlheinz has managed international clinical projects including an Influenza Program, spreading over three continents.
Team & Advisors
TeamRolf Naumann – Innovation & Funded ProjectsRolf joined Inamed GmbH in 2001 and Activaero GmbH in 2005, managing national and EU funding projects and scientific R&D projects. He coordinated the influenza project transfer to Activaero and is a certified Medical Product Advisor and Data Protection Representative. Lorraine Callaway – Clinical Team CoordinatorLorraine is an experienced Legal Secretary with over 15 years in business and administration. She has been managing the Trial Master Files for asthma and influenza studies. Since 2020, she has been the Clinical Team Coordinator at AspiAir. Karin Caspar – Office ManagementKarin is a qualified Industrial Business Management Assistant with over 25 years of experience. She has been the Personal Assistant to Executive Management at AspiAir since 2020. Axel Fischer – Business DevelopmentAxel is an experienced serial entrepreneur in the field of drug delivery systems and combination products. As CEO, Axel heads the Activoris Group Management Team. In his career, he held multiple management and board positions in life science companies and is co-founder of Actarmo Medical GmbH, SilviMed GmbH and Activoris Food Packaging GmbH. |
Scientific AdvisorsDr. Gerhard ScheuchDr. Scheuch is a Bio-Physicist with extensive experience in aerosol medicine and pulmonary drug delivery. He was President of ISAM and founder of Activaero, Inamed, and Ventaleon. His specialties include pulmonary deposition of aerosols and inhalation product development. Emilie HofstetterEmilie is the founder of HealthStrat Consulting, specializing in strategy and business development for disruptive medical technologies. She has worked at several pulmonary specialist startups and co-founded Atriva Therapeutics GmbH. She holds degrees in International Business and Political Sciences. Dr. Kirsten KaiserKirsten is the founder and CEO of Ratatoa Consulting GmbH, specializing in regulatory and clinical development. She was Executive VP at Skyepharma and began her career at Behringwerke. She holds an MD and PhD in hematology. Dr. Manfred FischerManfred is specializing in analytical, formulation, and device development. He began his career at AstraZeneca and held leadership positions at AltanaPharma and Eli Lilly. He played a key role in the development of Flutiform®, a combination inhaler used for the treatment of asthma. |
What we do
Just breathe! We transfer a well-known and safe i.v. therapy to the Patients’ Home.
We develop LASAG Inhale©, a first a first inhaled treatment for different types of pain. It is expected to have the advantages of Aspirin® i.v. (efficacy, safety and quick response), but can be applied by the patients themselves – at home and on the road.
More than 37 million people in the EU suffer from pain and migraine attacks, a considerable part of which have no access to adequate and effective medication. Today’s standard treatments are either costly or associated with side effects. Thus, many patients will benefit from a simple, inhaled treatment with a well-established drug.
Triptanes, the standard medication for migraine, have limited applicability and are not well tolerated by some patients; and novel antibody therapies (such as CGRP antagonists) are expensive (appr. US$ 800 per treatment).

the science
Acetylsalicylic acid (ASA): an effective and well-known treatment since decades
Migraine is a common disabling primary headache disorder. Epidemiological studies document its high prevalence and socio-economic impacts. In the Global Burden of Disease Study 2015, it was ranked the third-highest cause of disability. Some patients suffer from frequent migraines, up to several times a week, others only occasionally.
Acetylsalicylic acid (ASA) has been an effective and well-known treatment for acute migraine since years. Current labeling includes oral doses of ASA of up to 1000 mg for the treatment of acute attacks, the intravenous formulation is approved for injection for infusion of up to 1000 mg. The inhibition of COX-1 by ASA accounts for its inhibition of platelet aggregation, at the same time is also associated with adverse effects on the gastrointestinal tract when taken orally.
Applying drugs via inhalation is a usual and recommended procedure as the lung is an organ with high blood perfusion and an ideal entry point, and there is broad experience with inhaled ASA and its rapid bioavailability.
Diener HC et al. Efficacy and safety of intravenous acetylsalicylic acid lysinate compared to subcutaneous sumatriptan and parenteral placebo in the acute treatment of migraine. Cephalgia (1999) 19:581-8

the inhaler
At home or on the road!
Together with pulmonary drug delivery specialist Activoris, a high-performance mobile, battery-driven inhaler is being developed, allowing to nebulize high drug concentrations for deep lung deposition, thus keeping treatment times down. Further, a special and comfortable reconstitution and transfer system is under development, allowing a one-handed mixing and transfer of LASAG solution into the inhaler, making drug loading and inhaling a child’s play.

the study
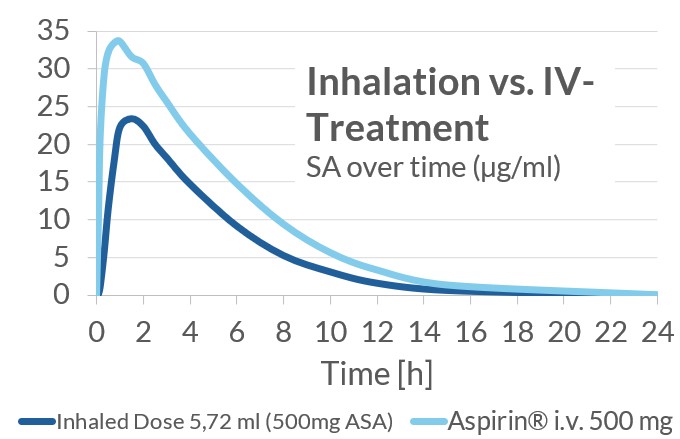
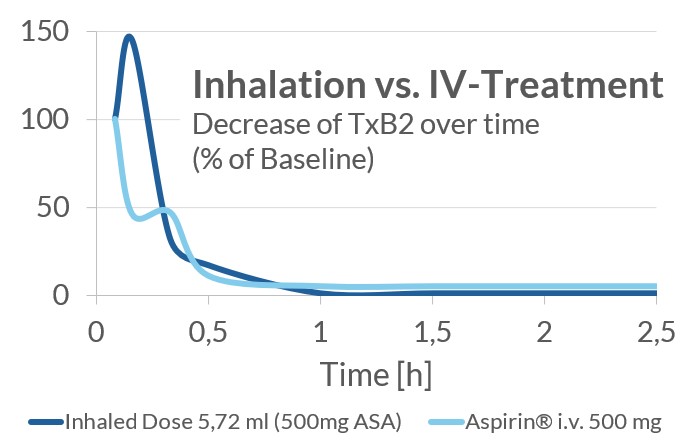
Clinical Data: Phase 1 PK-Study Results
The current study was a Phase 1 three dose level cross-over study, split in two parts. In the first part, single dose inhalations of LASAG were evaluated for PK/PD as well as safety and tolerability in 16 healthy subjects compared to i.v. LASAG in a cross-over design. In the second part, a single inhaled dose with a higher concentration of ASA/ml was evaluated in 12 healthy subjects.
As expected, a lower rate and extent of exposure with ASA was reached after inhaled administration of LASAG compared to intravenous administration. A clear and strong reduction in thromboxane B2 (TxB2) plasma concentration was seen for inhalation, comparable to intravenous treatment. TxB2 can serve as a surrogate marker for efficacy, because COX enzymes are involved in the production of thromboxanes and are responsible for the production of prostaglandins (key mediators in inflammation and pain responses). Inhalation was well-tolerated.

WHAT's New
Meet AspiAir at the following Events:
- Bio Europe Spring, March 17-19, 2025, Milan, Italy
- European Pain Federation EFIC, April 24-26, 2025, Lyon, France
- 2. Deutscher Kopfschmerzkongress, May 23-24, 2025, Cologne
WHERE WE ARE
Contact
AspiAir GmbH
Wohraer Straße 37
35285 Gemünden (Wohra)
Germany
Tel.: +49 (0) 6453 58530 40 Switch Board
Fax: +49 (0) 6453 58530 50
© 2025
Imprint
Disclaimer
Cookies


